Despite finding a beginning in the 1980s, 3D printing has taken some time to find its way into our everyday lives.
Now, between recent advances in technology and the expiry of a variety of key patents in the last few years, printers have never been cheaper, more accurate or more widespread.
With a larger audience, the applications for these devices have naturally become increasingly creative and surprising; from 3D printed foods such as chocolate, cheese or even entire pizzas, through to houses, backyard castles and some pretty wacky clothing, the technology is finding employment further and further afield from the manufacturing world.
But what about medicine?
Certainly, 3D printed medical devices and teaching aids have become commonplace, but what about printing living tissue for transplant into a human being?

3D printing a kidney structure. Image courtesy of the Wake Forest Institute for Regenerative Medicine.
Believe it or not, the basic technology for 3D printing organs and body parts has been around for some time, with patents for printing bone structures emerging from MIT as far back as 1993.
It wasn’t until 2003 however, when a team from Clemson University patented a method for ink-jetting living cells onto a substrate, that 3D printing tissues and 3D printing organs suddenly became a possibility.
In the ensuing years bioprinting technology has made some great leaps forward; researchers have not only managed to successfully produce human cartilage, bone, muscle and tissues from various organs, they have also successfully transplanted them into animals (including 3D printed ovaries allowing mice to reproduce).
Researchers at the Wake Forest Institute for Regenerative Health, long term pioneers in the bioprinting world, have created a machine that prints skin cells directly onto burn wounds, even going so far as to apply the correct type of skin cell based on wound depth. So when will 3D printing organs be common place in your local hospital?
Well, while rapidly producing transplant-ready organs is still a little way off, experts believe these breakthroughs form the foundation for just such a possibility and could, in even the next four or five decades, make it a reality.
There are already commercially available desktop bioprinters capable of 3D printing organs on the market.
So how does it work?
Though the technology is still very much in its infancy and faces many technical and ethical challenges before becoming available, there are already a variety of viable methods for printing tissues.
Despite this, the techniques share three primary requirements: a model to print, a 3D structure to provide support, and the cells themselves.
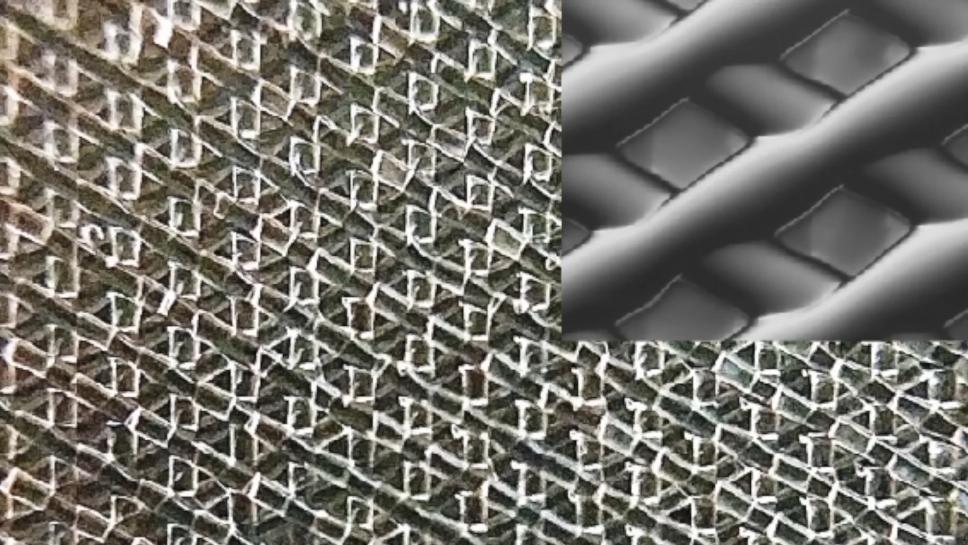
A scaffold architecture produced at Northwestern University, similar to that used to produce a functioning ovary.
Firstly, the model is generated either directly from the patient using CT and MRI scans, or through traditional CAD techniques. Constructed from cell-laden hydrogel, collagen or another biocompatible material, the models provide the shape and structure of the tissue around which the cells can grow. Either during this process or afterward, the model is seeded with a ‘bio-ink’ comprised of living cells from the patient in a nutrient-rich medium.
The cells can be taken directly from the target organ, such as the heart, kidney or skin, or stem cells can be used and differentiated afterwards. As they multiply and spread throughout the structure, microscopic channels or pores in the scaffold can provide the cells with the oxygen and nutrients they need to survive, whilst also forming the basis for blood vessels and a nervous system. Once the structure biodegrades, transplant ready tissue is left behind.
Once implanted, the body recognises the new tissue as part of itself and begins to integrate it, growing blood and nervous systems, cartilage and even bone around the new tissue in as little as five months.
This method of producing tissues for transplant brings with it some inherent benefits.
Problems with biocompatibility and organ rejection are largely avoided, as the transplant is made from the patient’s own cells.
Being able to generate organs from scratch could also vastly reduce transplant wait times, transform skin grafts and remove the need for animal and human test subjects entirely.
But why stop there?
With recent advances allowing control of stem cell differentiation in conjunction with 3D printing, and as the technology continues to improve, could a stem cell based printer produce entire limbs or organ systems in one go?
Could this extend a person’s lifespan? Perhaps we can take it further still and improve on our own evolution, printing denser muscles, stronger, lighter bones and ligaments, more efficient lungs or even entirely new organs allowing humans to survive and thrive in otherwise hostile environments.
Could modular humans be the future?

Dr Atala of the Wake Forest Institute holding a printed kidney structure during his TED talk.